Selective hydrogenation of 5-HMF to 2,5-DMF over a magnetically recoverable non-noble metal catalyst†
Abstract
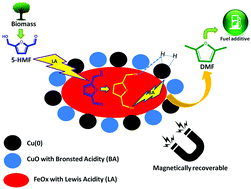
A non-noble bimetallic catalyst Cu–Fe (1 : 2) was magnetically recoverable, highly selective and efficient for 5-(hydroxymethyl) furfural (5-HMF) hydrogenation to 2,5-dimethyl furan (DMF). The structure–activity correlation was established by characterising the prepared catalyst by XRD, XPS, TEM, ESEM, BET surface area, N2-adsorption, NH3-TPD, pyridine-IR and H2-TPR measurements. The high catalytic efficiency was attributed to the oxophilic nature and Lewis acidity of Fe, whereas the selectivity towards DMF was attributed to the Brønsted acidity of CuO and its affinity towards the C–O bond which was further confirmed by NH3-TPD and Py-IR analyses. XPS and XRD revealed the presence of Cu/CuFe2O4 species which catalyzed the hydrogenolysis pathway. TEM and SEM images evidenced the presence of a Cu–Fe nanomorph in which Cu/CuFe2O4 was present. The size of the Cu–Fe nanomorph was found to be between 15.4–17.6 nm. Under the optimised reaction conditions, the highest conversion of 97% and selectivity of 93% were achieved.


 Please wait while we load your content...
Please wait while we load your content...