Hydrazo coupling: the efficient transition-metal-free C–H functionalization of 8-hydroxyquinoline and phenol through base catalysis†
Abstract
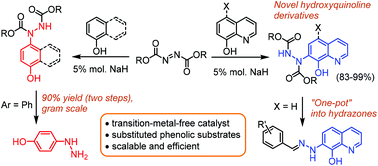
Azodicarboxylate esters are common reagents in organic synthesis laboratories due to their utility in the Mitsunobu reaction. They can also be regarded as possible starting compounds for C–H functionalization, which up until now has been mainly achieved by transition-metal-catalyzed reactions. We have developed a novel reaction involving the quantitative coupling of 8-hydroxyquinoline or phenol with azodicarboxylate esters. The functionalization proceeds under mild base-catalyzed conditions selectively, and either the ortho-position of 8-hydroxyquinoline or para-position of the phenol/naphthol is involved in the reaction. This type of transformation can be considered as “hydrazo coupling” (by analogy with azo coupling). Herein, we discuss a plausible mechanism for this catalyzed substitution, backing up our findings with deuterium NMR experiments and by varying the starting compounds and bases. Using Boc-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-Boc as a substrate, we have developed the convenient and efficient synthesis of (8-hydroxyquinolin-7-yl)hydrazines, as well as demonstrating a new stereoselective route for the synthesis of medicinally important 4-hydroxyphenylhydrazine for laboratory use, which almost doubles the yield of the common industrial process and reduces the number of synthetic steps. A new “one-pot” procedure for the synthesis of aromatic 8-hydroxyquinolin-7-yl hydrazones was applied.
N-Boc as a substrate, we have developed the convenient and efficient synthesis of (8-hydroxyquinolin-7-yl)hydrazines, as well as demonstrating a new stereoselective route for the synthesis of medicinally important 4-hydroxyphenylhydrazine for laboratory use, which almost doubles the yield of the common industrial process and reduces the number of synthetic steps. A new “one-pot” procedure for the synthesis of aromatic 8-hydroxyquinolin-7-yl hydrazones was applied.


 Please wait while we load your content...
Please wait while we load your content...