Selective hydrogenation via cascade catalysis on amorphous TiO2†
Abstract
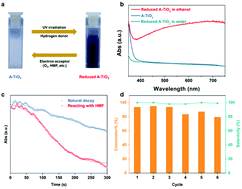
Photocatalysis arises as a green, sustainable approach to conventional industrial catalysis for the synthesis of valuable organic chemicals from biomass. However, photoinduced holes and/or reactive oxygen species are usually fatal to the selective redox process, and are responsible for inducing undesirable side reactions especially in the presence of water. Herein, we demonstrate a cascade strategy of photocatalytic oxidation/hydrogenation that could manage the efficient conversion (99%) of 5-hydroxymethylfurfural (HMF) to 2,5-bis(hydroxymethyl)furan (BHMF) with high selectivity (∼99%). The first reaction is conducted under UV irradiation, with the amorphous TiO2 surface storing the photoinduced electrons, and the protons extracted from alcohol via photooxidation. On subsequently switching to the dark, the stored H+/e− pairs undergo proton-coupled electron transfer (PCET) and contribute to the selective hydrogenation of HMF. This route effectively mitigates the negative impact of H2O and active species on the product selectivity. The oriented adsorption for the aldehyde group of HMF as well as the promoted product desorption bring about the high selectivity of BHMF over amorphous TiO2. The cascade catalytic pathway based on the stored H+/e− provides a reliable approach for biomass functionalization.


 Please wait while we load your content...
Please wait while we load your content...