Computational design of an intramolecular photocyclization reaction with state-selective reactivity: a strategy for indole synthesis†
Abstract
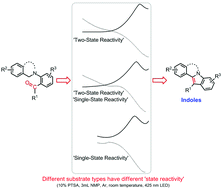
The development of a convenient and environmentally friendly indole synthesis method is of great methodological and pharmaceutical interest. In this work, we discovered an intramolecular photocyclization of carbonyls and tertiary amines for indole synthesis without relying on photocatalysts. A wide range of functional indoles were prepared with moderate to excellent yields under mild reaction conditions. Furthermore, our extensive quantum mechanics (QM) calculations and synthesis experiments revealed the ‘state-selective reactivity’ feature of this reaction, and different substrate types can achieve different yields through this feature. This new insight into the interplay between the ‘state-selective reactivity’ and the yields emphasizes its importance and gives guidance regarding the ‘state-selective reactivity’ for the high yield of various indole derivatives.


 Please wait while we load your content...
Please wait while we load your content...