Synthesis of rutaecarpine alkaloids via an electrochemical cross dehydrogenation coupling reaction†
Abstract
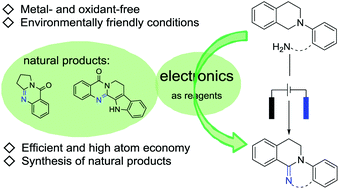
Substrates are directly oxidized at the anode without using any metal catalyst and oxidant to obtain a series of nitrogen heterocyclic compounds, including benzimidazoles and quinolinones. These compounds are highly tolerant to various functional groups and heterocycle-containing substrates. In addition, natural alkaloids, such as rutaecarpine and deoxyvasicinone, can be synthesized by this method.


 Please wait while we load your content...
Please wait while we load your content...