Binding enabled catalytic activation of SO2 by copper koneramine complexes under ambient conditions†‡
Abstract
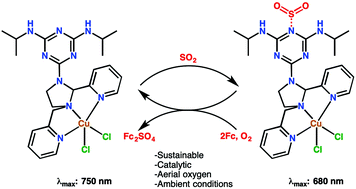
Reported is an unprecedented, simple and sustainable strategy to catalytically convert SO2 gas into sulfate dianions under ambient conditions utilizing ferrocene (SO2 + 2Fc + aerial O2 → Fc2SO4) and aerial oxygen, whereby ferrocenium sulfate was formed without any unwanted byproducts. The frequent urban haze and increase in respiratory health issues connected to the atmospheric SO2 concentration attracted interest in the sequestration and valorization of SO2. Even though a number of methods have been reported in the past decade for the stoichiometric sequestration and activation of SO2, effective catalytic activation of SO2 through homogeneous catalysis has not been realized before. The copper(II) koneramine complex [Cu(L-H)Cl2] was designed to have exclusive basic sites for SO2 binding, which complemented efficient electron transfer from ferrocene to SO2 that underwent S-oxygenation to yield sulfate dianions. In this proof of concept study, SO2 binding coupled electron transfer (SOCET) from ferrocene was established with the assistance of control experiments, electronic absorption spectroscopy, cyclic voltammetry and electrospray ionization mass spectrometry.


 Please wait while we load your content...
Please wait while we load your content...