Iridium-catalysed highly selective reduction–elimination of steroidal 4-en-3-ones to 3,5-dienes in water†
Abstract
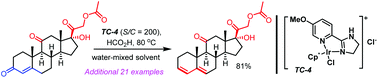
Steroidal 3,5-diene is an important structural motif in steroid drugs. In this report, an iridium-catalyzed reduction–elimination of readily available steroidal 4-en-3-ones is realized to prepare steroidal 3,5-dienes. At a low catalyst loading (S/C = 200), heating 4-en-3-ones in a water-mixed organic solvent with formic acid without inert atmosphere protection afforded the desired 3,5-dienes in moderate to excellent yields. In a gram-scale preparation, recrystallization is used instead of column chromatography to purify products. Excellent functionality tolerance and regioselectivity are featured. Structural moieties such as alkanols (primary, secondary and tertiary), esters (except for formate), tolylates, and ketones (endocyclic or exocyclic) are not affected. Surprisingly, the reduction–elimination only takes place at A-ring 4-en-3-ones. In addition, bicyclic 4-en-3-ones are also viable substrates. Synthetic applications of steroidal 3,5-dienes are demonstrated. Our method can also lead to steroidal 3,5-dienes-3-d (>99% D-incorporation) when DCO2D and D2O are used together.


 Please wait while we load your content...
Please wait while we load your content...