Visible-light-induced cascade radical ring-closure and pyridylation for the synthesis of tetrahydrofurans†
Abstract
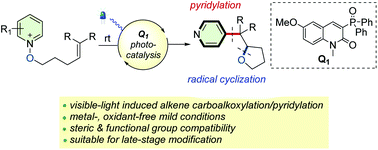
Reported is a novel visible-light-enabled alkoxy radical ring-closure and pyridylation from N-alkenyloxypyridinium salts serving as both alkoxy radical precursors and heteroaryl sources. This strategy features a photoredox tandem radical process involving a sequential fragmentation of an N-alkoxypyridinium salt, a radical cyclization process, and a pyridylation process. This method exhibited broad substrate scope, good functional group compatibility, and metal-free mild conditions, offering a powerful synthetic tool for assembling various pyridine-tethered tetrahydrofurans and late-stage functionalization of complex biorelevant molecules. Moreover, radical cascade cyclization could be successfully achieved, leading to the synthesis of previously challenging and important pyridine-tethered bicyclic oxaspiro ring systems.


 Please wait while we load your content...
Please wait while we load your content...