Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly(ethylene terephthalate) waste†
Abstract
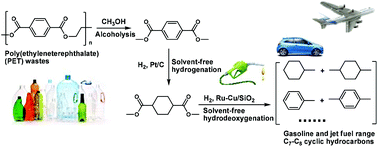
For the first time, gasoline and jet fuel range C7–C8 cycloalkanes and aromatics were selectively synthesized by the alcoholysis of poly(ethylene terephthalate) (PET) waste, followed by solvent-free hydrogenation and hydrodeoxygenation (HDO). It was found that methanol is highly reactive for the alcoholysis of PET waste. In the absence of any catalyst, a high yield of dimethyl terephthalate (97.3%) was achieved under mild conditions (473 K, 3.5 h). Dimethyl terephthalate exists as a solid and can be automatically separated from methanol with a decrease in temperature. Subsequently, dimethyl terephthalate was liquefied to dimethyl cyclohexane-1,4-dicarboxylate by hydrogenation over noble metal catalysts. Among the investigated catalysts, Pt/C exhibited the highest activity. Finally, the dimethyl cyclohexane-1,4-dicarboxylate as obtained was further hydrodeoxygenated to C7–C8 cycloalkanes and aromatics that can be used as gasoline or additives to improve the densities (or volumetric heat value) and sealabilities of current bio-jet fuels. Bimetallic Ru–Cu/SiO2 was found to be a promising HDO catalyst. According to the characterization results, the excellent HDO performance of Ru–Cu/SiO2 can be explained by the formation of smaller Ru–Cu alloy particles during the catalyst preparation. In real applications, dimethyl cyclohexane-1,4-dicarboxylate can also be simultaneously hydrodeoxygenated with biomass derived oxygenates to produce jet fuel with a suitable content of cycloalkanes and aromatics.


 Please wait while we load your content...
Please wait while we load your content...