Fully alternating sustainable polyesters from epoxides and cyclic anhydrides: economical and metal-free dual catalysis†
Abstract
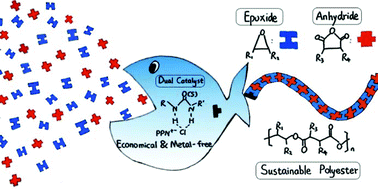
We disclose an economical and metal-free catalyst system of (thio)ureas/organic bases for effective fully alternating copolymerization of epoxides and anhydrides, which remains a challenge in sustainable polyester synthesis. A series of commercial (thio)ureas and organic bases were employed as dual catalysts to optimize the activity, and the results indicate that the (thio)ureas with higher basicity paired with quaternary onium salts show higher activity. Particularly, the U1/PPNCl pair shows extremely high TOF (456 h−1) toward the ring-opening alternating copolymerization of cyclohexane oxide with phthalic anhydride, which is comparable to the activity of metal-based complex catalysts. Structurally diverse polyesters with high ester linkages (99%) and various glass transition temperatures (ranging from −8 to 133 °C) can be obtained by coupling of various renewable epoxides and anhydrides. What's more, structural analysis of the resultant polymers and the interactions between the monomers and the dual catalysts confirmed by 1H NMR reveal that the (thio)urea can act as a bifunctional catalyst, which activates both the initiating chain end and the monomer through H-bonding in the (thio)urea/PPNCl catalyst system, resulting in high activity.


 Please wait while we load your content...
Please wait while we load your content...