Donor–acceptor preassociation, excited state solvation threshold, and optical energy cost as challenges in chemical applications of photobases†
Abstract
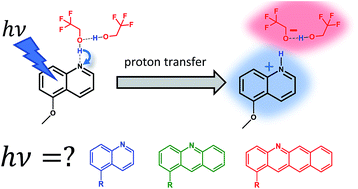
Photobases are molecules with increased pKa in the excited state that can serve to transduce light energy into proton removal capability. They can be used to control chemical reactions using light, such as removing protons from a catalytic site in reactions that are rate-limited by proton transfer. We identify and explore several major challenges toward their practical applications. Two important challenges are the need for pre-association (or ground state hydrogen bonding) between the proton donor and the photobase, and the need for excited state solvation of the photogenerated products. We investigate these two challenges with the photobase 5-methoxyquinoline as the proton acceptor and a low-pKa alcohol, 2,2,2-trifluoroethanol, as the proton donor. We vary the concentration of the donor in a background non-hydrogen-bonding solvent. Using absorption spectroscopy, we have identified that the donor–acceptor concentration ratio must exceed 100 : 1 to achieve appreciable ground state hydrogen bonding. Interestingly, emission spectroscopy reveals that the onset of ground state hydrogen bonding does not guarantee successful excited state proton transfer. It takes an additional order of magnitude increase in donor–acceptor ratio to achieve that goal, revealing that it is necessary to have excess donor molecules to reach the solvation threshold for the photogenerated products. The next challenge is reducing the large ground-excited state energy gap, which often requires UV photons to drive proton transfer. We show experimental and computational data comparing the photobasicity and optical energy gap for a few N-aromatic heterocyclic photobases. In general, we find that reducing the energy gap by increasing the conjugation size necessarily reduces photobasicity, while adding substituents of varying electron-withdrawing strength allows some fine-tuning of this effect. The combination of these two factors provide a preliminary design space for creating new photobasic molecules.
- This article is part of the themed collection: Ultrafast photoinduced energy and charge transfer


 Please wait while we load your content...
Please wait while we load your content...