Ultrafast flow chemistry for the acid-catalyzed conversion of fructose†
Abstract
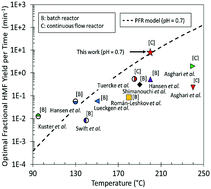
Biomass conversion to chemicals and fuels has been epitomized by batch processing and long reaction times that limit the economic viability of future biorefineries and prevent modular manufacturing near the source-production site that is necessary due to the biomass’ large water content. We introduce a continuous flow microreactor to enable process intensification and one thousand-fold device miniaturization for remote chemical processing. We demonstrate the approach in HCl-catalyzed fructose dehydration in water. We characterize and quantify the mixing using laser induced fluorescence (LIF) of fluorescent dyes and particle image velocimetry (PIV) of fluorescent microspheres. The estimated mixing times ranged from 0.03 to 4.8 s for residence times from 1 to 120 s, respectively. Moreover, a curved channel geometry induces secondary (Dean) vortices that produce a three-fold increase in mixing by diffusion through advection along the cross-sectional direction of the channel, creating dissipative chaotic mixing under laminar flow. We then obtain for the first time isothermal kinetics of fructose dehydration at short contact times and high temperatures and compare to a previously published, hybrid ab initio and data-driven kinetic model. Rigorous model optimization is carried out and an optimal HMF yield of 54% is attained in a single aqueous phase. High temperatures and low pH improve the reactor throughput by increasing the HMF space-time yield. We demonstrate the highest ever productivity (HMF yield per unit time). Importantly, this can be achieved at ∼100% fructose conversion, eliminating the need for recycling to improve economics. Furthermore, optimization of the reactor dimensions and the specific energy dissipation rate results in ∼60% pressure-drop related energy savings. We describe a methodology to scale-up microreactors to millimeter size and increase productivity 16-fold under optimal conditions. The energy efficiency and cost analysis for HMF production from corn stover of a typical corn farm indicate an optimal strategy for numbering-up millimeter size reactors and, importantly, they underscore the potential feasibility of producing bioproducts in a distributed manner.


 Please wait while we load your content...
Please wait while we load your content...