A graphite intercalation compound associated with liquid Na–K towards ultra-stable and high-capacity alkali metal anodes†
Abstract
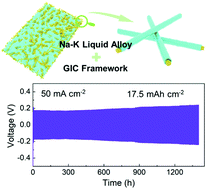
Alkali metal anodes (Li, Na, K) are highly promising for enabling high-energy-density rechargeable batteries due to their high theoretical capacities and low redox potentials. Although extensive studies have been performed on Li-metal, dendrite growth and serious interface issues are still fundamental challenges for practical alkali metal batteries (AMBs). Here, we report an in situ-formed graphite intercalation compound (GIC) framework that enables Na–K liquid alloy to be used in ultra-stable and high-capacity anodes, attributed to the synergy of fast electron and mass transport of the GIC networks associated with the self-healing behavior of the Na–K alloy. The Na–K composite electrode is highly stable; it sustains repeated stripping/deposition over 5000 hours at 20 mA cm−2 and achieves stable electrodeposition even at 80 mA cm−2 and 16 mA h cm−2. When coupled with various cathodes, versatile AMBs are realized with long cycling and high operating voltages. This framework electrode design presents new insight into developing dendrite-free alkali metal anodes.


 Please wait while we load your content...
Please wait while we load your content...