Stabilization of different redox levels of a tridentate benzoxazole amidophenoxide ligand when bound to Co(iii) or V(v) †
Abstract
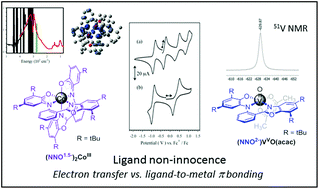
A tridentate benzoxazole-containing aminophenol ligand NNOH2 was coordinated to Co and V metal centers and the electronic structure of the resultant complexes characterized by both experimental and theoretical methods. The solid state structure of the Co complex exhibits a distorted octahedral geometry with two tridentate ligands bound in meridional fashion, and coordination-sphere bond lengths consistent with a Co(III) oxidation state. EPR and magnetic data support a S = 1/2 ground state, and a formal electronic description of Co(III)(NNOAP)(NNOISQ) where NNOAP corresponds to an amidophenoxide and NNOISQ to the iminosemiquinone redox level. However, the metrical parameters are similar for both ligands in the solid state, and DFT calculations support delocalization of the ligand radical over both ligands, affording an intermediate ligand redox level Co(III)(NNO1.5−)(NNO1.5−). The vanadyl complex exhibits a distorted octahedral geometry in the solid state consistent with a V(V) metal center and amidophenoxide (NNOAP), acetylacetonate and oxo ligands. The ligand metrical parameters are consistent with significant amidophenoxide to V(V) π donation. Overall, our results highlight the roles of electron transfer, delocalization, and π bonding in the metal complexes under study, and thus the complexity in assignment of the electronic structure in these systems.


 Please wait while we load your content...
Please wait while we load your content...