Boron complexes of aromatic 5-substituted iminopyrrolyl ligands: synthesis, structure, and luminescence properties†
Abstract
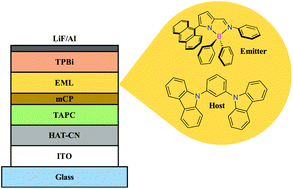
A group of new mononuclear boron chelate compounds [BPh2{κ2N,N′-5-R-NC4H2-2-C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar}] (R = Ar = C6H57; R = C6H5, Ar = 2,6-iPr2C6H38; R = Anthracen-9-yl (Anthr), Ar = C6H59; R = Anthr, Ar = 2,6-iPr2C6H310) were synthesized via the reaction of B(C6H5)3 with the corresponding 5-substituted 2-(N-arylformimino)pyrrole ligand precursors 3–6. These complexes were prepared in order to evaluate the luminescence potential derived from the substitution of the position 5 of the pyrrolyl ring with an aromatic group. Compounds 7–10 were photophysically characterized in solution and in the solid state. The 5-phenyl-2-iminopyrrolyl-BPh2 complexes 7 and 8 are blue emitters and have enhanced photoluminescence quantum yields in the solid state (ΦPL) up to 0.95, whereas the 5-anthracenyl derivatives 9 and 10 have green-bluish fluorescence and a ΦPL of 0.49 and 0.24, respectively. DFT and TDDFT studies were performed, considering the effect of solvent and dispersion, in order to show how the geometries of compounds 7–10 changed from the ground to the excited state, to assign electronic transitions, and to rationalize the observed luminescence. These materials were applied in organic light-emitting diodes (OLEDs), with various device structures, the best showing an external quantum efficiency of 2.75% together with a high luminance of 23 530 cd m−2.
N–Ar}] (R = Ar = C6H57; R = C6H5, Ar = 2,6-iPr2C6H38; R = Anthracen-9-yl (Anthr), Ar = C6H59; R = Anthr, Ar = 2,6-iPr2C6H310) were synthesized via the reaction of B(C6H5)3 with the corresponding 5-substituted 2-(N-arylformimino)pyrrole ligand precursors 3–6. These complexes were prepared in order to evaluate the luminescence potential derived from the substitution of the position 5 of the pyrrolyl ring with an aromatic group. Compounds 7–10 were photophysically characterized in solution and in the solid state. The 5-phenyl-2-iminopyrrolyl-BPh2 complexes 7 and 8 are blue emitters and have enhanced photoluminescence quantum yields in the solid state (ΦPL) up to 0.95, whereas the 5-anthracenyl derivatives 9 and 10 have green-bluish fluorescence and a ΦPL of 0.49 and 0.24, respectively. DFT and TDDFT studies were performed, considering the effect of solvent and dispersion, in order to show how the geometries of compounds 7–10 changed from the ground to the excited state, to assign electronic transitions, and to rationalize the observed luminescence. These materials were applied in organic light-emitting diodes (OLEDs), with various device structures, the best showing an external quantum efficiency of 2.75% together with a high luminance of 23 530 cd m−2.


 Please wait while we load your content...
Please wait while we load your content...