Abstract
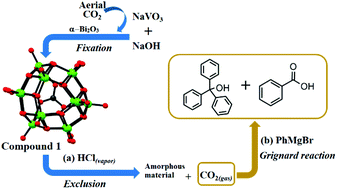
An aqueous synthesis, involving the reduction of the VO3− anion in a mild alkaline pH in the presence of α-Bi2O3, led to the formation of a fully reduced polyoxovanadate (POV) capsule, with CO32− anion encapsulation in its internal cavity, in the compound [Na6(H2O)24][H8VIV15O36(CO3)]·3N2H4·10H2O (1). This CO32− anion encapsulation, the source of which is absorbed aerial CO2 in the pertinent aqueous alkaline reaction mixture, occurs only in the presence of α-Bi2O3. Compound 1 crystals, upon exposure to HCl acid vapor, exclude CO2 gas that can react with the Grignard reagent (PhMgBr) to form triphenylcarbinol and benzoic acid; during this solid–vapor interface reaction, compound 1 itself transforms into an amorphous material that includes the Cl− anion but could not be characterized unambiguously. Thus, we have synthesized a chloride ion (Cl−) encapsulated compound [Na10(H2O)24][H3VIV15O36(Cl)]·6H2O (2) in a direct synthesis protocol, which has been characterized by crystallography as well as by other spectroscopic methods. Compounds 1 and 2, each having fifteen vanadium(IV) centers, exhibit interesting magnetism in their solid states. The temperature-dependent magnetic susceptibilities for compounds 1 and 2 have been recordred at 0.1 T in the temperature range of 3–300 K. The temperature-dependent magnetic susceptibilities of compounds 1 and 2 are shown in the form of χMvs. T and their product χMT vs. T plots.


 Please wait while we load your content...
Please wait while we load your content...