Field-induced single-ion magnet behaviour of a hexacoordinated Co(ii) complex with easy-axis-type magnetic anisotropy†
Abstract
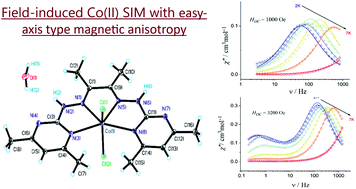
A coordination compound with the composition [CoLCl2]·H2O (L = bis-condensation product of diacetyl and 2-hydrazinyl-4,6-dimethylpyrimidine) was synthesized, in which the Co(II) ion was hexacoordinated. Under applied DC fields, this compound exhibited single-ion magnet behavior. Two relaxation processes were observed when increasing the applied magnetic field from 1000 to 3200 Oe. The first relaxation (high-frequency) was observed both at 1000 Oe and 3200 Oe, while the second relaxation was only registered under a field of 3200 Oe at low frequencies (<1 Hz) and low temperatures (<5 K). Modeling of the magnetic DC properties using the Griffith Hamiltonian accompanied by quantum chemical calculations revealed easy-axis-type magnetic anisotropy with weak rhombic contributions.


 Please wait while we load your content...
Please wait while we load your content...