Computational study of H2 binding to MH3 (M = Ti, V, or Cr)†
Abstract
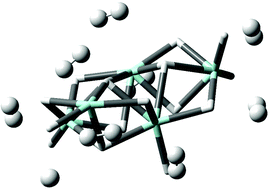
A series of amorphous materials based on hitherto elusive early transition metal hydrides MH3 (M = Ti, V, and Cr) and capable of binding H2via the Kubas interaction has shown great promise for hydrogen storage applications, approaching US DoE system targets in some cases [Phys. Chem. Chem. Phys., 2015, 17, 9480; Chem. Mat., 2013, 25, 4765; J. Phys. Chem. C, 2016, 120, 11407]. We here apply quantum chemical computational techniques to study models of the H2 binding sites in these materials. Starting with monomeric MH3 (M = Ti, V, and Cr) we progress to M2H6 and then pentametallic systems, analyzing the H2 binding geometries, energies, vibrational frequencies and electronic structure, finding clear evidence of significant Kubas binding. Dihydrogen binding energies range from 22 to 53 kJ mol−1. In agreement with experiment, we conclude that while TiH3 binds H2 exclusively through the Kubas interaction, VH3 and CrH3 additionally physisorb dihydrogen, making these more attractive for practical applications.


 Please wait while we load your content...
Please wait while we load your content...