Electrophilic boron carboxylate and phosphinate complexes†
Abstract
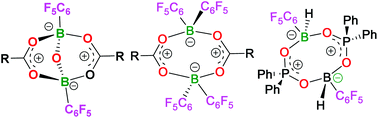
The reactions of a series of carboxylic acids with H2B(C6F5)·SMe2 are shown to afford species of the form [RC(O)OB(C6F5)]2O, (R = Tol 1, Ph 2, C6F53, Me2BrC 4, Me 5) in 87–95% yields with the concurrent reduction of the carboxylic acid to the corresponding aldehyde. A mechanism for the formation of 1–5 is proposed to proceed via a cyclic eight-membered ring species. Analogues of these species were prepared via reactions of carboxylic and phosphinic acids with HB(C6F5)2 and H2B(C6F5)·SMe2, respectively, to give [TolC(O)OB(C6F5)2]26, [(C6F5)C(O)OB(C6F5)2]27, and [Ph2P(O)OBH(C6F5)]28. These products react subsequently to give TolC(O)OBH(C6F5)(NC5H4NMe2) 9 and Ph2P(O)OBH(C6F5)(NC5H4NMe2) 10. The acyloxyborate derivatives 1–4 were shown to be inactive in mediating the direct amidation of carboxylic acids, consistent with previous observations that infer the need for a sterically congested environment about the boron centres.


 Please wait while we load your content...
Please wait while we load your content...