Facile synthesis of 1,5-disubstituted 1,2,3-triazoles by the regiospecific alkylation of a ruthenium triazolato complex†
Abstract
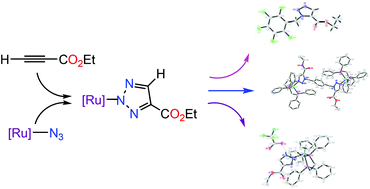
The alkylation of the N(2)-bound ruthenium triazolate [Ru]N3C2HCO2Et (2, [Ru] = (η5-C5H5)(dppe)Ru, dppe = Ph2PCH2CH2PPh2) with benzylbromides is reported. The regiospecific alkylation of 2, which results from the [3 + 2] cycloaddition of ethyl propiolate with [Ru]-N3 (1), gives a series of cationic N(1)-bound N(3)-alkylated-4-ethoxycarbonyl triazolato complexes {[Ru]N3(CH2R)C2HCO2Et}[Br] (3a, R = 4-CH2Br-C6H4; 3b, R = 3,5-(CH2Br)2-C6H3; 3c, R = 2,6-F2-C6H3; 3d, R = 4-CN-C6H4) and the subsequent cleavage of the Ru–N bond of 3a–3d gives N(1)-alkylated-5-ethoxycarbonyl triazoles N3(CH2R)C2HCO2Et (4a–4d) and [Ru]-Br, which, on reacting with sodium azide, would afford [Ru]-N3 (1) thus forming a reaction cycle. The treatment of {[Ru]N3(CH2C6F5)C2HCO2Et}[Br] (3e) with sodium azide in refluxing ethanol gives the free triazole N3(CH2C6F5)C2HCO2Et (4e) and 1. The treatment of 2 with an equivalent of 3a affords a dinuclear bis(triazolato) complex {α,α′-bis([Ru]N3C2HCO2Et)-p-xylene}[Br]2 (5) and an organic bis(triazole) complex α,α′-bis(N3C2HCO2Et)-p-xylene (6). The treatment of 2 with CF3COOH in CHCl3 at room temperature affords a mixture of N(2)-bound 1H- and 3H-4-ethoxycarbonyl triazolato complexes {[Ru]N3HC2HCO2Et}[CF3COO] (1H-7) and (3H-7) in a ratio of 5 : 2. The structures of 4e, 5 and 1H-7 were confirmed by single-crystal X-ray diffraction analysis.


 Please wait while we load your content...
Please wait while we load your content...