Ce3+ self-doped CeOx/FeOCl: an efficient Fenton catalyst for phenol degradation under mild conditions†
Abstract
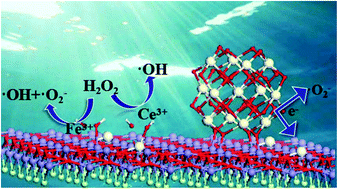
Herein, a novel Ce3+ self-doped CeOx/FeOCl composite was successfully prepared by a facile method for the first time, which showed remarkable catalytic activity as a Fenton catalyst in the degradation of phenol under the conditions of a neutral solution, room temperature and natural light. In CeOx/FeOCl, 5.23% CeOx is the optimal condition, and the degradation constant (k) of CeOx/FeOCl is greater than that of FeOCl by a factor of 10.8. CeOCl in the composite plays a more important role than CeO2, which greatly increases the production of ˙OH radicals. Furthermore, the Ce-doping in FeOCl accelerates the separation efficiency of the photogenerated electron–hole pairs. The increased surface area and surface potential of CeOx/FeOCl than those of FeOCl effectively promote the adsorption of phenol, which is 4.05 times that of FeOCl. According to the DFT calculations, the Ce-doping in FeOCl enhances the structural stability by increasing the strength of the chemical bonds. The adsorption of H2O2 with Ce3+ is energetically favorable, which promotes the production of ˙OH radicals. A synergistic mechanism for the enhanced catalytic performance of CeOx/FeOCl is proposed.


 Please wait while we load your content...
Please wait while we load your content...