Syntheses, structures, magnetism and electrocatalytic oxygen evolution for four cobalt, manganese and copper complexes with dinuclear, 1D and 3D structures†
Abstract
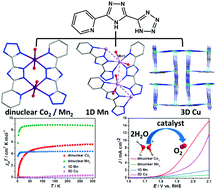
Four 3-(tetrazol-5-yl)-5-(pyrid-2-yl)-1,2,4-triazole (H2TPT) based complexes of [Co2(TPT)2(H2O)2] (1), [Mn2(TPT)2(H2O)2] (2), [Mn(TPT)(H2O)2]n (3) and [Cu(TPT)]n (4) have been solvothermally synthesized and structurally characterized by X-ray single-crystal diffraction analysis. Complexes 1 and 2 display isostructural dinuclear structures, while complex 3 exhibits a 1D zigzag chain structure. The structural difference for 2 and 3 may be caused by 100 and 160 °C temperature-controlled conditions. Complex 4 is a 3D framework structure in which the Cu2+ ion is in square pyramid coordination geometry. Complexes 1–4 display good thermal stability evaluated by thermogravimetric analysis. Complexes 1 and 4 show very strong antiferromagnetic interactions. The electrocatalytic oxygen evolution of complexes 1–4 was tested under neutral conditions, which revealed that the four complexes possess electrocatalytic oxygen evolution activity. Complex 1 exhibits a current density of 10.0 mA cm−2 at a potential of 2.00 V (vs. RHE), presenting 50-fold improvement in specific activity over the glassy carbon electrode.


 Please wait while we load your content...
Please wait while we load your content...