Theoretical insights into intermolecular interactions during d8 organometallic self-aggregation†
Abstract
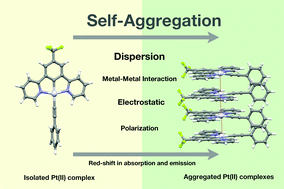
Metallophilicity was once considered as the major driver of aggregation in metal–organic complexes. However, the contributions of other intermolecular interactions cannot be neglected. We performed a theoretical study on the intermolecular interactions involved in the aggregation of a series of planar organoplatinum(II) complexes with different substituents. The structures, thermodynamic properties and electronic structures of Pt-complex monomers and oligomers were investigated, and the roles played by Pt–Pt interaction, dispersion, and electrostatics were discussed. The results indicate that the strongest attractive interactions among the Pt-complexes are dispersion interactions. Metal–metal interactions are present on a relatively small scale compared to the interactions with ligands, whereas they affect the frontier molecular orbitals notably. During the aggregation of such Pt(II) complexes, thermodynamically stable aggregates with larger interaction energies are preferentially constructed. Next, aggregates with greater electrostatic attraction and smaller dipole moments tend to be formed.


 Please wait while we load your content...
Please wait while we load your content...