A quantitative study of vapor diffusions for crystallizations: rates and solvent parameter changes†
Abstract
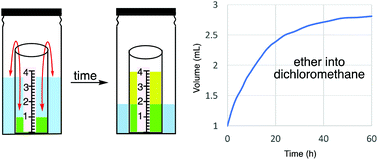
Vapor diffusion crystallizations are among the most versatile methods for growing X-ray quality crystals. While many experimental sections describe the successful use of various solvent combinations, the literature has been entirely lacking in quantitative data (rates, measures of solvent strength changes) that might allow more informed planning rather than simple trial-and-error approaches. We here report the diffusion-induced volume changes for 44 solvent combinations over the first 60 h under standardized conditions, plus six more combinations that exhibit little or no volume changes. Additionally, the inner and outer vial compositions at 24 h were determined, and the resulting changes in solvation parameters were quantified using Hansen solubility parameters. Some general preliminary effects of changes in volume ratios and scale are described. These results identify two dozen solvent combinations with larger changes in solvent parameters than the very commonly used diethyl ether/dichloromethane example. These results should allow a more informed approach to the execution of vapor diffusion crystallizations than has previously been possible.


 Please wait while we load your content...
Please wait while we load your content...