Mono- and bimetallic pentacoordinate silicon complexes of a chelating bis(catecholimine) ligand†
Abstract
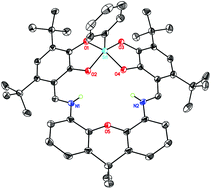
Schiff base condensation of 4,5-diamino-9,9-dimethylxanthene with 4,6-di-tert-butylcatechol-3-carboxaldehyde affords the bis(catecholimine) ligand XbicH4, which can bind metals in both a square bis(catecholate) upper pocket and a pentagonal N2O3 lower pocket. Metalation with PhSiCl3 results in [(XbicH2)SiPh][HCl2], where the silicon adopts a five-coordinate, square pyramidal geometry in the upper pocket and the lower pocket binds to two protons on the imine nitrogens. Deprotonation of the imines with LiOtBu, NaN[SiMe3]2, or AgOAc results in binding of the univalent metal ion in the lower pocket, where it adopts an unusual pentagonal monopyramidal geometry in the solid state. The complexes show irreversible electrochemistry, with oxidations taking place at relatively high potentials.


 Please wait while we load your content...
Please wait while we load your content...