Synthesis of well-defined yttrium-based Lewis acids by capturing a reaction intermediate and catalytic application for cycloaddition of CO2 to epoxides under atmospheric pressure†
Abstract
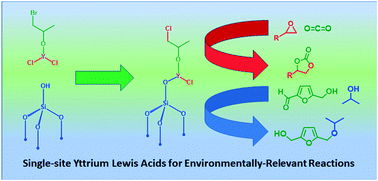
Despite widespread use of yttrium halide complexes as powerful Lewis acids in catalysis, no strategies have yet been developed to prepare well-defined heterogeneous systems. Herein, we show that by applying the methodology of surface organometallic chemistry (SOMC), a readily available intermediate of the mechanism of the cycloaddition of CO2 to epoxides catalyzed by YCl3/TBAB (TBAB: tetrabutylammonium bromide) can be grafted on silica resulting in a well-defined complex [(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO–)YCl(–OCH(CH3)CH2Cl)]. The complex was thoroughly characterized by means of elemental analysis, FT-IR, solid state (SS) NMR, XPS and XANES techniques. The thus-prepared surface complex serves as heterogeneous Lewis acid for the cycloaddition of CO2 to several epoxides under atmospheric pressure performing as a simple but efficient and recyclable material. Remarkably, the isolated complex prepared on highly dehydroxylated silica performed as the most efficient compound. Additional catalytic studies show that the yttrium complexes prepared in this study have the potential to be employed also as versatile Lewis acid catalyst for 5-hydroxymethyl furfural (HMF) reductive etherification. DFT calculations were carried out to investigate the possible grafting pathways and the mechanistic pathways of CO2-epoxide cycloaddition catalyzed by different surface model complexes.
SiO–)YCl(–OCH(CH3)CH2Cl)]. The complex was thoroughly characterized by means of elemental analysis, FT-IR, solid state (SS) NMR, XPS and XANES techniques. The thus-prepared surface complex serves as heterogeneous Lewis acid for the cycloaddition of CO2 to several epoxides under atmospheric pressure performing as a simple but efficient and recyclable material. Remarkably, the isolated complex prepared on highly dehydroxylated silica performed as the most efficient compound. Additional catalytic studies show that the yttrium complexes prepared in this study have the potential to be employed also as versatile Lewis acid catalyst for 5-hydroxymethyl furfural (HMF) reductive etherification. DFT calculations were carried out to investigate the possible grafting pathways and the mechanistic pathways of CO2-epoxide cycloaddition catalyzed by different surface model complexes.


 Please wait while we load your content...
Please wait while we load your content...