Fast and deep oxidative desulfurization of dibenzothiophene with catalysts of MoO3–TiO2@MCM-22 featuring adjustable Lewis and Brønsted acid sites†
Abstract
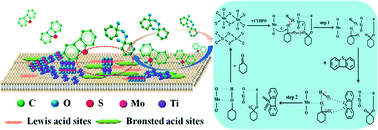
The synthesis of high-performance and recyclable catalysts for oxidative desulfurization (ODS) from fuels has been a significant challenge. In this study, novel catalysts of MoO3–TiO2@MCM-22 with excellent catalytic performance were successfully prepared via a facile impregnation method. The results showed that the optimal catalyst at the Mo–Ti mass ratio of 1 : 4 (MT-1 : 4) exhibited highest catalytic efficiency for the ODS of dibenzothiophene (DBT) with a sulfur conversion of 99.96% within 15 min. Interestingly, the enhanced ODS activity was attributed to the synergistic effect between MoO3 and TiO2, which was achieved by adjusting the concentrations of Lewis and Brønsted acid sites on the surface of MCM-22. The catalyst MT-1 : 4 achieved the highest concentrations of Lewis and Brønsted acid sites and the largest combined index, resulting in the formation of peroxometallate complexes. Moreover, the kinetic studies revealed that the ODS was a pseudo first-order reaction with an apparent activation energy of 48.9 kJ mol−1. There was no significant reduction in the catalytic activity after 8 successive cycles, which manifested the perfect reusability of the catalyst MT-1 : 4 for the ODS system. Furthermore, a plausible ODS mechanism was proposed using the MoO3–TiO2@MCM-22 catalyst. Therefore, the prepared MoO3–TiO2@MCM-22 catalyst exhibited favorable industrial application potential for ODS.


 Please wait while we load your content...
Please wait while we load your content...