Turning the product selectivity of nitrile hydrogenation from primary to secondary amines by precise modification of Pd/SiC catalysts using NiO nanodots†
Abstract
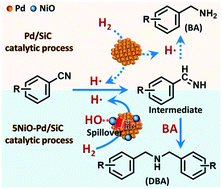
The selectivity of supported metal catalysts is mainly determined by the active metallic component, and thus turning the selectivity to a completely different product is rarely achieved by modification of the catalysts. Hydrogenation of nitriles is an efficient and environmentally benign route for the synthesis of valuable amines, but it usually produces mixtures of primary, secondary and even tertiary amines. Herein we report that the selectivity of Pd/SiC catalysts for the hydrogenation of nitriles with H2 can be turned from primary to secondary amines by modification of NiO nanodots. In the modified catalysts, the NiO nanodots act as reactive sites to consume hydrogen radicals on the Pd surface, and thus prolong the lifetime of an imine intermediate that determines the product selectivity. Under mild conditions (30 °C, atmospheric H2), Pd/SiC and NiO–Pd/SiC catalysts exhibit high selectivity to primary (94%) and secondary (99%) amines, respectively.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...