Improved photo-dechlorination at polar photocatalysts K3B6O10X (X = Cl, Br) by halogen atoms-modulated polarization†
Abstract
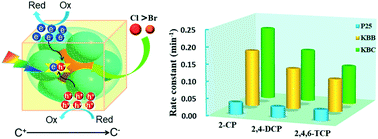
The contamination of chlorophenols in water has been considered as an urgent environmental issue. In this study, two polar photocatalysts with a similar structure, K3B6O10Br (KBB) and K3B6O10Cl (KBC) were synthesized through a high temperature solid phase method and applied for the effective mineralization and removal of the chlorophenols. More than 92.0% of 2-chlorophenols can be fully degraded within 16 min under UV light irradiation, which is six times faster than the commercial P25 TiO2 catalyst. The out-of-center distortions capability of the XK6 octahedrons is greatly influenced by X (X = Cl and Br) atoms. The magnitude of the out-of-center distortion of KBC and KBB were 0.137 and 0.027, respectively. A better photocatalytic activity performance was therefore observed for KBC due to its higher spontaneous polarization ability and facile charge separation process. Kelvin probe force microscopy (KPFM) experiments further proved KBC has a larger surface potential change before and after the light irradiation. Reactive species capture experiments confirmed the role of radicals in the order of ˙O2− > e− > h+ > ˙OH.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...