A mononuclear tantalum catalyst with a peroxocarbonate ligand for olefin epoxidation in compressed CO2†
Abstract
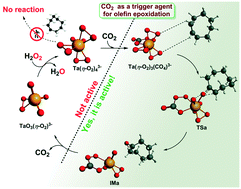
A new class of tantalum-based peroxocarbonate ionic liquid ([P4,4,4,4]3[Ta(η2-O2)3(CO4)]) has been generated through the reaction of pressurized CO2 with [P4,4,4,4]3[Ta(O)3(η2-O2)] in the presence of H2O2 during the reaction process. The newly formed species has been verified by NMR, FT-IR, HRMS and density functional theory (DFT) calculations. The CO2-induced monomeric peroxocarbonate anion-based ionic liquid is more advantageous than the monomeric peroxotantalate analogue for the epoxidation of olefins under very mild conditions. Interestingly, the transformation between peroxotantalate and peroxocarbonate species is completely reversible, and CO2 can actually act as a trigger agent for epoxidation reaction. The further mechanism studies by DFT calculation reveal that peroxo η2-O2 (site a) affords higher reactivity towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond than that of peroxocarbonate–CO4 (site b). These quantitative illustrations of the relationship between structural properties and kinetic consequences enable rational design for an efficient and environmental IL catalyst for the epoxidation of olefins.
C bond than that of peroxocarbonate–CO4 (site b). These quantitative illustrations of the relationship between structural properties and kinetic consequences enable rational design for an efficient and environmental IL catalyst for the epoxidation of olefins.


 Please wait while we load your content...
Please wait while we load your content...