Mechanistic study of ethanol steam reforming on TM–Mo6S8 clusters: a DFT study†
Abstract
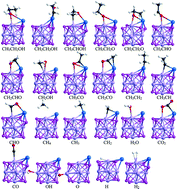
The mechanism of ethanol steam reforming (ESR) on TM–Mo6S8 (TM = Pt, Pd) clusters is systematically investigated using a combination of the microscopic kinetic model, energetic span model (ESM) and d-band model under density functional theory (DFT) calculations. The results show that Pd–Mo6S8 shows the best performance both in terms of activity and stability, due to facile H2 evolution from the cluster and less carbon coking. ESR is initiated by decomposition of ethanol: CH3CH2OH* → CH3CH2O* → CH3CHO* → CH3CO* + H* → CH3* + CO* → CH4* + CO*, followed by the water-gas shift (CO* + H2O* → CO2* + H2*) reaction (WGSR) to produce CO2 and H2; the rate-determining step is the O–H bond scission of OH species. In other words, the WGSR, not the ethanol decomposition, is the bottleneck for the overall ethanol steam reforming process. Our results provide a mechanism and insight into the importance of hydroxyl groups to the ESR reaction over Pd–Mo6S8 catalysts, which may shed light on designing improved catalysts and is beneficial to the H2 formation.


 Please wait while we load your content...
Please wait while we load your content...