Water mediated oxygen activation in NH3 SCR reaction over a Cu-SAPO-34 catalyst: a first principles study†
Abstract
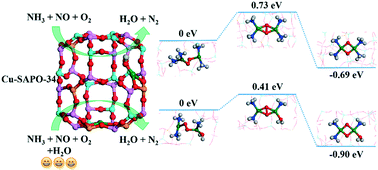
At low temperature, water can greatly improve the NH3 selective catalytic reduction (SCR) performance of Cu-SAPO-34 under standard reaction conditions. During the SCR process on Cu-CHA catalysts, oxygen (O2) activation over pairs of Cu(NH3)2+ complexes is a crucial step. The concentration of H2O in the reaction atmosphere may affect the local environment of Cu ions and subsequent oxygen activation. In this work, first-principles calculations are used to study the adsorption of NH3 on Cu sites and the detailed reaction paths of O2 activation with or without water in Cu-SAPO-34. NH3 adsorption is generally favored over H2O on Cu ions, yet at low temperature and with partial pressure of water vapor, H2O and NH3 molecules could co-adsorb on the same Cu site from the phase diagram analysis. During the O2 activation process, this kind of behavior will influence the formation of the Cu(NH3)2+ complex and a new kind of Cu(NH3)(H2O)+ complex will form during the reduction process of Cu2+ ions. Compared with O2 activation over pairs of Cu(NH3)2+ complexes, Cu(NH3)2+–Cu(NH3)(H2O)+ complexes change the torsion angle of the Cu–O–O–Cu configuration and decrease the reaction energy barriers of O2 activation in Cu-SAPO-34. Our results shed light on the water mediated NH3 SCR reaction mechanism of Cu-SAPO-34 at the molecular level.


 Please wait while we load your content...
Please wait while we load your content...