Remote ion-pair interactions in Fe-porphyrin-based molecular catalysts for the hydrogen evolution reaction†
Abstract
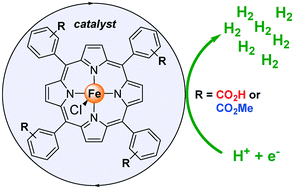
The environmentally benign production of clean energy is extremely important for the sustainable progress of our society. In this respect, dihydrogen (H2) has been considered in the last decades as an efficient energy carrier and much effort has been directed to the hydrogen evolution reaction (HER). Herein, we report on the efficiency of iron-based 5,10,15,20-tetraphenylporphyrins containing carboxylate groups in different positions (ortho, meta and para of the meso-substituted aryl groups of the porphyrin backbone) as molecular catalysts for the HER. The iron-based porphyrin containing the carboxylic acids in the ortho position was found completely inactive in the HER. Furthermore, besides stereoelectronic control, the subtle differences observed in the cyclic voltammograms (CV) as well as those associated with the electrocatalytic activity might involve a previously neglected ion-pair interaction between the carboxylate groups of the porphyrin scaffold and the chloride anions belonging to the proton source, which highlights the relevance of ion-pair contacts remote from the active center for this type of catalytic system.


 Please wait while we load your content...
Please wait while we load your content...