Catalytic activity of palladium-doped silver dilute nanoalloys for formate oxidation from a theoretical perspective†
Abstract
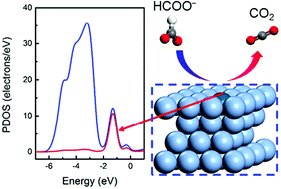
The large-scale practical application of formate oxidation reaction (FOR) catalysts is hindered by their low activity and high cost. Herein, for the first time, a series of Pd-doped Ag dilute nanoalloys is demonstrated to have high catalytic activity in FOR with reduced consumption of Pd metals through density functional theory calculations, where the effects of potential, solvent and spin on catalytic performance are discussed. The Pd1Ag(111) single-atom alloy (SAA) exhibits higher FOR catalytic activity as reflected by the low limiting potential of 0.026 eV for the direct association path and a value of 0.084 eV for the direct dissociation path, and the lowest activation energy of 0.774 eV for the rate-determining-step in the direct dissociation path compared with Pd2Ag(111) and Pd3Ag(111) dilute alloys. Pd1Ag(111) SAA exhibits an extremely narrow sharp peak in the partial density of states from −0.75 to −2.0 eV, which is due to the free-atom-like electronic structure of the single Pd atom. The isolated Pd single atom is more stable by −0.041 and −0.097 eV, respectively, than the aggregated Pd2 and Pd3 atom clusters on the Ag(111) surface, which verifies the potential application of Pd1Ag(111) SAA in experiments. Overall, this work further elucidates the theoretical profile of FOR and provides a new strategy for designing the catalytic reaction at the atomic level.


 Please wait while we load your content...
Please wait while we load your content...