Effects of water vapor on the reaction of CH2OO with NH3†
Abstract
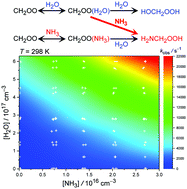
The reaction of the simplest Criegee intermediate, CH2OO, with ammonia and water vapor has been investigated at 278–308 K and under 100–760 Torr by monitoring the strong UV absorption of CH2OO. We found that the observed decay rate of CH2OO becomes much larger when ammonia and water vapor are both present; the combinational effect of ammonia and water vapor is significantly greater than the sum of their individual contributions, revealing a strong synergic effect. The kinetic data are consistent with a termolecular process of CH2OO + NH3 + H2O reaction, of which the reaction rate coefficient was determined to be kNH3+H2O = (8.2 ± 1.2) × 10−31 cm6 s−1 at 298 K with a negative activation energy, Ea = −8.0 ± 0.8 kcal mol−1 [kNH3+H2O(T) = 1.04 × 10−36 exp(4047/T)]. Quantum chemistry calculation (at the QCISD(T)/aug-cc-pVTZ//B3LYP/6-311+G(2d,2p) level) found a low-energy reaction pathway, on which water accepts a hydrogen atom (or proton) from ammonia and releases another hydrogen atom to the terminal oxygen of CH2OO. The predicted products are H2NCH2OOH and a new H2O molecule, indicating water catalysis. This reaction is very fast and probably barrierless, which poses a theoretical challenge to modeling the related kinetics.


 Please wait while we load your content...
Please wait while we load your content...