Assessment of van der Waals inclusive density functional theory methods for adsorption and selective dehydrogenation of formic acid on Pt(111) surface†
Abstract
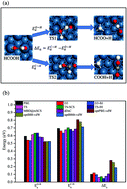
In this work, we studied the adsorption and catalytic dehydrogenation of formic acid (HCOOH) on Pt(111) surface using different van der Waals inclusive density functional theory (DFT) methods. Our results indicate that the PBE + dDsC method has the best overall performance on the description of adsorption and catalytic selectivity. We found the improved van der Waals (vdW) corrected methods (PBE + D3, PBE + TS, PBE + TS-SCS, PBE + TS/IH, PBE + MBD@rsSCS, and PBE + dDsC) and optimized vdW functionals (optPBE-vdW, optB88-vdW, and optB86b-vdW) perform well to estimate the adsorption energies of HCOOH and HCOO molecules on Pt(111) surface. The vdW-inclusive DFT approaches as well as the conventional PBE functional predict a higher activation barrier for C–H breaking by comparison of O–H breaking in the selective dehydrogenation of formic acid. However, the optimized vdW functionals evidently underestimate the rate constant of C–H breaking reaction, and then fail to describe the catalytic selectivity of the HCOOH's dehydrogenation. Both PBE + dDsC and PBE predict a similar temperature dependence of the ratio of reaction rate constants for O–H breaking versus C–H breaking, though PBE functional underestimate the adsorption energies.


 Please wait while we load your content...
Please wait while we load your content...