Li-ion hopping conduction in highly concentrated lithium bis(fluorosulfonyl)amide/dinitrile liquid electrolytes†
Abstract
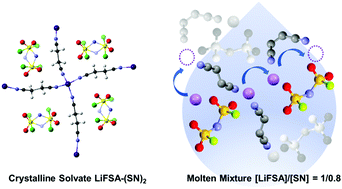
Li+ ion hopping conduction in highly concentrated solutions of lithium bis(fluorosulfonyl)amide (LiFSA) dissolved in dinitrile solvents, namely succinonitrile, glutaronitrile, and adiponitrile, was investigated. Phase behaviors of the LiFSA/dinitrile binary mixtures assessed by differential scanning calorimetry suggested that LiFSA and the dinitriles form stable solvates in a molar ratio of 1 : 2. For succinonitrile, a glass forming room temperature liquid is formed when [LiFSA]/[succinonitrile] > 1. The corresponding glutaronitrile and adiponitrile mixtures have melting points below 60 °C. The self-diffusion coefficients of Li+, FSA−, and dinitrile measured with pulsed field gradient NMR suggested that Li+ ion diffuses faster than anion and dinitrile in the liquids of composition [LiFSA]/[dinitrile] = 1/0.8, indicating emergence of Li+ ion hopping conduction. X-ray crystallography for the LiFSA–(dinitrile)2 solvates and Raman spectroscopy for the liquids with composition [LiFSA]/[dinitrile] > 1 revealed that the two cyano groups of the dinitrile coordinate to two different Li+ ions and form solvent-bridged structures of (Li+–dinitrile–Li+). In addition, the Raman spectra suggested that ionic aggregates (Li+–FSA−–Li+) are formed in the liquids with composition [LiFSA]/[dinitrile] > 1. Although there is frequent ligand (dinitrile and/or anion) exchange for each Li+ ion in the liquid state, the polymeric network structures (solvent-bridged structure and ionic aggregates) restrict the facile motion of ligands because each ligand is interacting with multiple Li+ ions in the highly concentrated electrolytes. This induces the faster diffusion of the Li+ ion than that of the ligands, i.e., hopping conduction of Li+ through ligand exchange. Electrochemical measurements clarified that the [LiFSA]/[succinonitrile] = 1/0.8 electrolyte possesses a relatively high Li+ transport ability (limiting current density > 7 mA cm−2) thanks to the Li+ hopping conduction, regardless of its extremely high viscosity (3142 mPa s) and relatively low conductivity (0.26 mS cm−1) at room temperature. Furthermore, this electrolyte was shown to have a high Li+ transference number (>0.6), exhibited reversible Li metal deposition/dissolution i.e. suppression of reductive decomposition of the solvent, and could be successfully applied to graphite and LiNi1/3Mn1/3Co1/3O2 half-cells.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...