Molecular dynamics simulations reveal the mechanism of graphene oxide nanosheet inhibition of Aβ1–42 peptide aggregation†
Abstract
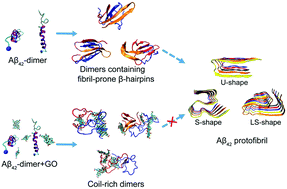
The aggregation of the amyloid-beta (Aβ) peptides into toxic β-sheet-rich oligomers, protofibrils and mature fibrils is the major pathological hallmark of Alzheimer's disease (AD). Inhibiting the β-sheet formation and fibrillization of Aβ peptides is considered an important treatment strategy for AD. Graphene oxide (GO) has attracted particular interest in the anti-aggregation of amyloid proteins due to its good ability of crossing the blood–brain barrier (BBB), low cytotoxicity and good biocompatibility. Recent experiments reported that GO nanosheets could strongly inhibit the fibril formation of Aβ1–42 and reduce its cytotoxicity. However, the mechanism of suppressing Aβ1–42 fibrillization by GO nanosheets is not well understood. Aβ1–42 dimer is the smallest toxic oligomer of Aβ1–42 aggregation. As a starting step to understand the inhibitory effect of GO nanosheets towards Aβ1–42 aggregation, we investigated the conformational distribution of the Aβ1–42 dimer with or without GO nanosheets by performing explicit-solvent replica exchange molecular dynamics simulations. Our simulations showed that Aβ1–42 peptides could form diverse β-sheet rich dimeric conformations, whereas those conformations were significantly inhibited after the addition of GO nanosheets. We found that GO suppressed the β-sheet formation of Aβ1–42 mostly by weakening inter-peptide interactions mostly via salt bridge, hydrogen bonding and cation–π interactions with charged residues D1, E3, R5, D7, E11, K16, E22, K28 and A42. The π–π and hydrophobic interactions between GO and Aβ1–42 also play a role in the inhibition of Aβ aggregation. This study provides mechanistic insights into Aβ1–42 aggregation and amyloid inhibition by GO nanosheets, which may provide new clues for the development of therapeutic candidates against AD.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...