Hydration and ion association of aqueous choline chloride and chlorocholine chloride†
Abstract
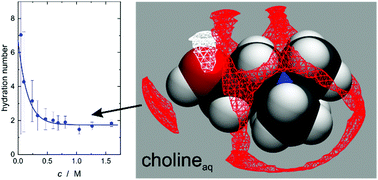
The choline ion (Ch+) is ubiquitous in nature and also its synthetic homologue, chlorocholine (ClCh+), is widely used. Nevertheless, surprisingly little information on the hydration and counter-ion binding of these cations can be found in the literature. In this contribution we report effective hydration numbers, determined by dielectric relaxation spectroscopy, and ion-pair association constants with Cl−, determined by dilute-solution conductivity measurements. In combination with RISM calculations the obtained data suggest that for Ch+ water is bound to the hydroxy group via hydrogen bonds whereas for ClCh+ a rather stiff clathrate-like shell around the chlorine atom seems to be formed. With Cl− both cations form contact ion pairs with association constants of only ∼2 to 3 M−1.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...