Energetics of exciton binding and dissociation in polythiophenes: a tight binding approach†
Abstract
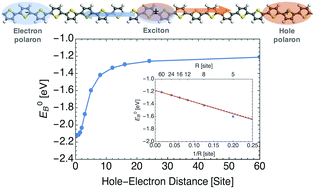
Organic photovoltaics offer a potential low-cost alternative to inorganic solar cells. Crucial to the performance of these devices is the generation of free charges, which occurs through the dissociation of excitons. Here we study excitons in polythiophenes, their stability and energetics of dissociation and separation into charge carriers. Excitons are excited electron and hole pairs bound by Coulomb interactions. To separate into unbound charges, the exciton binding energy must be overcome. We use a tight binding Hamiltonian to describe the exciton binding energy and its dissociation potential, for an exciton confined to a single polymer chain. Our model accounts for polaronic effects, arising from reorganization of nuclei and from polarization of the surrounding dielectric, which stabilize the separated carriers and thereby affect the exciton dissociation potential. We examine the effects of an applied electric field on the dissociation potential, and relate the field strength necessary to unbind the hole–electron pair to the maximum attractive Coulomb force between them. We apply our model to study the exciton at a donor–acceptor interface on a block-copolymer. Interfacial polarization alters the exciton binding potential, rendering the hole–electron pair easier to unbind.


 Please wait while we load your content...
Please wait while we load your content...