Mechanistic insights into a non-heme 2-oxoglutarate-dependent ethylene-forming enzyme: selectivity of ethylene-formation versusl-Arg hydroxylation†
Abstract
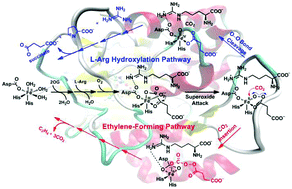
The ethylene-forming enzyme (EFE) is a unique member of the Fe(II)- and 2-oxoglutarate-dependent (Fe/2OG) oxygenases. It converts 2OG into ethylene plus three CO2 molecules (ethylene-forming reaction) and also catalyzes the C5 hydroxylation of L-arginine coupled to the oxidative decarboxylation of 2OG (L-Arg hydroxylation reaction). To uncover the mechanisms of the dual transformations by EFE, quantum mechanical/molecular mechanical (QM/MM) calculations were carried out. Based on the results, a branched mechanism was proposed. An FeII-peroxysuccinate complex with a dissociated CO2 generated through the nucleophilic attack of the superoxo moiety of the Fe–O2 species on the keto carbon of 2OG is the key common intermediate in both reactions. A competition between the subsequent CO2 insertion (a key step in the ethylene-forming pathway) and the O–O bond cleavage (leading to the formation of succinate) governs the product selectivity. The calculated reaction barriers suggested that the CO2 insertion is favored over the O–O bond cleavage. This is consistent with the product preference observed in experiments. By comparison with the results of AsqJ (an Fe/2OG oxygenase that leads to substrate oxidation exclusively), the protein environment was found to be crucial for the selectivity. Further calculations demonstrated that the local electric field of the protein environment in EFE promotes ethylene formation by acting as a charge template, exemplifying the importance of the electrostatic interaction in enzyme catalysis. These findings offer mechanistic insights into the EFE catalysis and provide important clues for better understanding the unique ethylene-forming capability of EFE compared with other Fe/2OG oxygenases.


 Please wait while we load your content...
Please wait while we load your content...