Voltammetric demonstration of thermally induced natural convection in aqueous solution†
Abstract
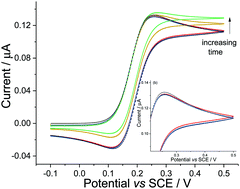
In electrochemical systems imperfect thermostating inevitably leads to the presence of bulk convective flows. As recognised by Nernst [Z. Phys. Chem., 1904, 52] damping of these bulk convective flows next to a solid surface, or at the electrode, leads to diffusional mass transport predominating locally. This work questions the exclusivity of diffusional transport and provides hitherto unexplored physical insights into how thermally induced flows in bulk solution can, on both macro- and microelectrodes, influence a voltammetric measurement. Imperfect thermostating results in flows in the bulk solution which are predicted and here expeimentally shown to be of the order of 100 μm s−1. Here we show that even in the absence of natural convective flows induced by the electrochemical reaction itself, this thermally induced bulk convection can significantly affect the voltammetric response. First, evaporative losses from an open electrochemical cell can be sufficient to produce convective flows that can alter the electrochemical response. Second, electrodes with various sizes and geometries have been investigated and experimental results evidence that the sensitivity of an electrode to these flows in bulk solution is to a large extent controlled by the size of the surrounding non-conductive supporting substrate used to insulate parts of the electrode.


 Please wait while we load your content...
Please wait while we load your content...