Selective enhanced sampling in dihedral energy facilitates overcoming the dihedral energy increase in protein folding and accelerates the searching for protein native structure†
Abstract
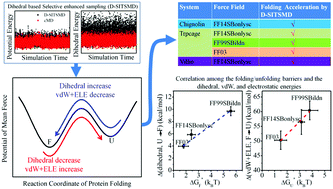
The dihedral energy function is the most influential parameter in molecular mechanics (MM) force field parameter optimization. A selective enhanced sampling of dihedral energy could effectively reflect the influence of dihedral energy settings on protein secondary structure representation, which in turn testifies the availability of the force field in folding simulation. Here, a Dihedral-based Selective Integrated-Tempering-Sampling Molecular Dynamics (D-SITSMD) simulation method is shown to provide a selective enhanced sampling of dihedral energy without introducing large energetic noise. Its capabilities of searching for protein natively folded structure and providing the underlying folding pathway are evaluated through the folding tests of three peptides (chignolin, TC5b, and HP35) with multiple AMBER force fields (FF14SBonlysc, FF99SBildn, or FF03) and the comparison to presented experimental data and REMD simulations. Both above-mentioned capabilities are improved, displaying the potential of D-SITSMD in the studies of in silico protein folding and structure refinement. Additionally, it is commonly observed among the test simulation systems that their folding processes are thermodynamically favorable for non-bonded vdW and electrostatic energies but unfavorable for dihedral energy, such that the folding barrier height correlated with the dihedral energy increase from the unfolded to folded state whereas the unfolding free energy barrier correlated with the combined increase of vdW and electrostatic energies in the unfolding process. It is speculated that the influence of a force field on the folding barrier of a protein is fulfilled mainly through regulating the contribution of dihedral energy to determine the secondary structure formation in the global folding process.


 Please wait while we load your content...
Please wait while we load your content...