Li-Ion solvation in propylene carbonate electrolytes determined by molecular rotational measurements
Abstract
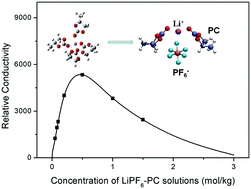
Lithium-ion batteries are an attractive power source for a wide variety of applications. Expanding the performance limit of current Li-ion batteries requires ion–solvent interaction, which governs the ion transport behavior of electrolytes, to be fully understood. We herein examine the coordination number of the Li+ ion in LiPF6–PC solutions using femtosecond vibrational spectroscopy. Surprisingly, we found that the coordination number of PC in the first solvation shell of Li+ decreases from 4 to 2 as the salt concentration increases. At dilute salt concentrations, the Li(PC)4+ complex with a tetrahedral geometry is dominant, while at high salt concentrations, the presence of anions in the first solvation shell modifies the solvation structure, leading only 2 PC molecules to coordinate to Li+ directly. The variety of the solvation structure provides a rational explanation for the ionic conductivity changing as the salt concentration increases.


 Please wait while we load your content...
Please wait while we load your content...