Using hydrogenated and perfluorinated gases to probe the interactions and structure of fluorinated ionic liquids†
Abstract
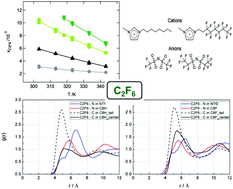
After studying the properties of a mixture of hydrogenated and fluorinated ionic liquids we have measured the solubility of perfluoromethane, perfluoroethane and perfluoropropane in 1-alkyl-3-methylimidazolium based ionic liquids with hydrogenated or fluorinated alkyl side-chains: 1-octyl-3-methylimidazolium bis[trifluoromethylsulfonyl]amide ([C8C1Im][NTf2]), 1-octyl-3-methylimidazolium bis[pentafluoroethylsulfonyl]amide ([C8C1Im][BETI]), 1-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)-3-methylimidazolium bis[trifluoromethylsulfonyl]amide ([C8H4F13C1Im][NTf2]), and 1-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)-3-methylimidazolium bis[pentafluoroethylsulfonyl]amide ([C8H4F13C1Im][BETI]). The ionic liquids expand on mixing and mix endothermally with a relatively high enthalpy of mixing (ΔmixH for [C8C1Im]x[C8H4F13C1Im](1−x)[NTf2] of ca. 0.85 kJ mol−1 for x = 0.5) when compared with other ionic mixtures. The solubility of the perfluorinated gases is larger in the fluorinated ionic liquids when compared with that of their hydrogenated counterparts and follows the order [C8H4F13C1Im][BETI] > [C8H4F13C1Im][NTf2] > [C8C1Im][BETI] > [C8C1Im][NTf2], a behaviour explained by a slightly more favourable enthalpy of solvation. The fluorinated ionic liquids nevertheless do not dissolve larger quantities of perfluorinated gases than their hydrogenated equivalents, as observed by comparing the results herein for perfluoroethane to those measured previously for ethane in the same ionic liquids. By using molecular simulations to study the microscopic structure of the solutions, we could show that the gases, hydrogenated and fluorinated, are always preferentially solvated in the apolar domains of the ionic liquids, and the hydrogenated hydrocarbon gases are always more soluble, independent of the fluorination of the ionic liquid.


 Please wait while we load your content...
Please wait while we load your content...