Theoretical insights into the formation and stability of radical oxygen species in cryptochromes†
Abstract
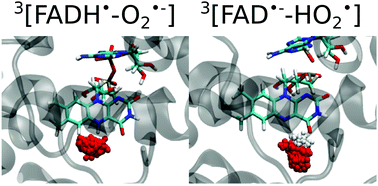
Cryptochrome is a blue-light absorbing flavoprotein containing a flavin adenine dinucleotide (FAD) cofactor. FAD can accept up to two electrons and two protons, which can be subsequently transferred to substrates present in the binding pocket. It is well known that reactive oxygen species are generated when triplet molecular oxygen is present in the cavity. Here, we investigate the formation and stability of radical oxygen species in Drosophila melanogaster cryptochrome using molecular dynamics simulations and electronic structure calculations. We find that the superoxide and hydroxyl radicals in doublet spin states are stabilized in the pocket due to the attractive electrostatic interactions and hydrogen bonding with partially reduced FAD. These findings validate from a molecular dynamics perspective that [FAD˙−–HO2˙] or [FADH˙–O2˙−] can be alternative radical pairs at the origin of magnetoreception.


 Please wait while we load your content...
Please wait while we load your content...