Strong influence of weak hydrogen bonding on actinide–phosphonate complexation: accurate predictions from DFT followed by experimental validation†
Abstract
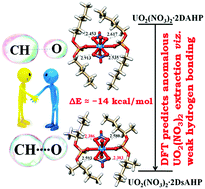
Among the varied classes of weak hydrogen bond, the CH⋯O type is one of immense interest as it governs the finer structures of biological and chemical molecules, hence determining their functionalities. In the present work, this weak hydrogen bond has been shown to strongly influence the complexation behaviour of uranyl nitrate [UO2(NO3)2] with diamyl-H-phosphonate (DAHP) and its branched isomer disecamyl-H-phosphonate (DsAHP). The structures of the bare ligands and complexes have been optimized by density functional theory (DFT) calculations. Surprisingly, despite having the same chemical composition the branched UO2(NO3)2·2DsAHP complex shows a remarkably higher stability (by ∼14 kcal mol−1) compared to the UO2(NO3)2·2DAHP complex. Careful inspection of the optimized structures reveals the existence of multiple CH⋯O hydrogen-bonding interactions between the nitrate oxygens or U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygens and the α-hydrogens in the alkyl chains of the ligands. Comparatively stronger such bonds are found in the UO2(NO3)2·2DsAHP complex. The binding free energies associated with the complexes are computed and favoured superior binding energetics for the more stable UO2(NO3)2·2DsAHP complex. Calculations involving diisoamyl-H-phosphonate (DiAHP) and its complexes have also been performed. Theoretical predictions are experimentally tested by carrying out the extraction of U(VI) from nitric acid media using these ligands. DAHP, DsAHP and DiAHP are synthesised, characterised by NMR and evaluated for their physicochemical properties viz. viscosity, density and aqueous solubility. It was experimentally discovered that indeed DsAHP complexation with uranyl nitrate is more favoured. H-phosphonates are generically classified as acidic extractants owing to the formation of an enol tautomer at lower acidities, hence complexing the metal ion by proton exchange. Our experiments interestingly reveal a neutral ligand characteristic for DsAHP alone which is generically an acidic extractant. Furthermore, the enol tautomer of H-phosphonates that governs their extraction profiles at low acidities is also explored by DFT and the anomalous pH dependent complexation trend of DsAHP could be successfully explained. The extractions of Pu(IV) and Th(IV) have also been carried out in addition to U(VI). Solvent extraction behaviour of Am(III) was also studied with all three ligands; the positive binding energies computed for the Am(III) complexation corroborate with our experimental results on the poor extraction of Am(III).
O oxygens and the α-hydrogens in the alkyl chains of the ligands. Comparatively stronger such bonds are found in the UO2(NO3)2·2DsAHP complex. The binding free energies associated with the complexes are computed and favoured superior binding energetics for the more stable UO2(NO3)2·2DsAHP complex. Calculations involving diisoamyl-H-phosphonate (DiAHP) and its complexes have also been performed. Theoretical predictions are experimentally tested by carrying out the extraction of U(VI) from nitric acid media using these ligands. DAHP, DsAHP and DiAHP are synthesised, characterised by NMR and evaluated for their physicochemical properties viz. viscosity, density and aqueous solubility. It was experimentally discovered that indeed DsAHP complexation with uranyl nitrate is more favoured. H-phosphonates are generically classified as acidic extractants owing to the formation of an enol tautomer at lower acidities, hence complexing the metal ion by proton exchange. Our experiments interestingly reveal a neutral ligand characteristic for DsAHP alone which is generically an acidic extractant. Furthermore, the enol tautomer of H-phosphonates that governs their extraction profiles at low acidities is also explored by DFT and the anomalous pH dependent complexation trend of DsAHP could be successfully explained. The extractions of Pu(IV) and Th(IV) have also been carried out in addition to U(VI). Solvent extraction behaviour of Am(III) was also studied with all three ligands; the positive binding energies computed for the Am(III) complexation corroborate with our experimental results on the poor extraction of Am(III).


 Please wait while we load your content...
Please wait while we load your content...