Oxidative addition of carbon dioxide into mesoionics†
Abstract
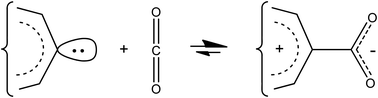
This work examines the prospect of making stable mesoionic compounds of the type mesomeric betaine R+–CO2− from direct oxidative additions of carbon dioxide to suitably-delocalized singlet carbene moieties, with bold objectives of carbon sequestration and overall energy storage. A set of possible candidates for such mesoionic compounds is theoretically explored through DFT calculations, inspecting coupling paths, thermodynamic and kinetic stabilities, and geometric and electronic structural features. Among others, the addressed cationic parts include aromatic rings in their broader sense, phenalene systems, and odd linear polyenic chains. Various structurally-close neutral alternatives such as oxiranones or carbene-acid forms are also considered. In the linear polyenic chain family, there is stark contrast between 4N + 1 and 4N − 1 lengths, with ensuing substantial consequences for stabilities and structures. Amino substitutions can favor mesoionic arrangements through their cation-stabilizing π-donor properties, further supported by possible strong intramolecular hydrogen bonds, but they can also contribute to weaken their kinetic stability through the existence of stable neutral imino alternatives. All in all, mesoionics including tropylium, phenalene, or 4N + 1 odd polyene frames as cationic parts could be reasonable targets.


 Please wait while we load your content...
Please wait while we load your content...