Thermodynamics and the potential energy landscape: case study of small water clusters†
Abstract
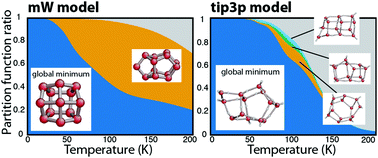
We investigated the structure and the thermodynamic properties of small water clusters with the nested sampling computational technique, using two different water models, the coarse-grained mW (up to 25 molecules) and the flexible version of the TIP3P (up to 16 molecules). By mapping the entire potential energy landscape of the clusters, we calculated the heat capacity curves, located the structural transitions and identified those local minimum basins which contribute the most to the total partition function. We found that in the case of the mW model, trends in first-order-like and continuous-like transitions can be very well matched to the characteristics of the landscape: cluster sizes with fewer and narrower local minimum basins show a sharper ‘melting’ peak on the heat capacity curve. Trends in the case of the TIP3P model were not easily assigned to the changing occupation of basins, and the contribution of local minima was negligible, except for n = 7, 15 and 16.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...