Surface termination of MgB2 unveiled by a combination of adsorption experiments and theoretical calculations†
Abstract
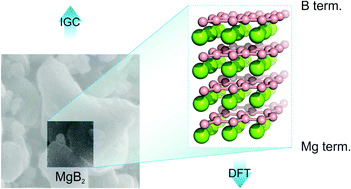
Superconductivity in polycrystalline and thin-film MgB2 is strongly affected by the termination of its surface, but a reliable determination of the surface termination is still a challenging task of surface chemistry. Here, the surface properties of superconducting MgB2 were investigated using a combination of inverse gas chromatography and van der Waals corrected density functional theory calculations. The dispersive surface energy was measured as a function of the surface coverage and its value (58 mJ m−2 to 48 mJ m−2) was verified by high-level non-local EXX + RPA calculations, which predicted that the dispersive contribution to the cleavage energy was 56 mJ m−2. The isosteric adsorption enthalpies of cyclohexane, dioxane, acetone and acetonitrile molecules were measured on an MgB2 sample and compared to the DFT calculated enthalpies for the Mg-terminated MgB2, B-terminated MgB2 and MgO(001) surfaces. The close agreement between theory and experiment for the Mg-terminated surface suggested that the magnesium termination is the dominant surface phase of MgB2. Thus, combining inverse gas chromatography experiments with theoretical calculations may provide information about the surface termination.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...